
“Hey it’s a bottle of vitamin D here, still looking for a justification for you to buy me.”
Few vitamins continue to disappoint like vitamin D supplementation. But the same could be argued for vitamin research in general: Observational trials find correlations between low levels of, or consumption of, specific vitamins, and a long list of maladies. But when actually studied prospectively, in clinical trials, the evidence comes up short. Again and again and again. Supplementing your diet with vitamins remains popular not because of the evidence, but despite the evidence. Sales of vitamins continue to grow (and have been accelerated by COVID) despite evidence that they are neither necessary nor beneficial for the majority of people that take them.
It is important to note that vitamin supplementation is not pseudoscience. There are some circumstances where vitamin supplementation may be beneficial. While medically significant deficiencies of vitamins are generally rare in developed countries (you don’t see scurvy or rickets much anymore), dietary restrictions, pregnancy, and even socio-economic status may increase the possibility of a deficiency, and in those situations, supplementation may be evidence based. The million (billion) dollar question is whether supplementation in the absence of any clear medical need is advisable or necessary. As a pharmacist I have spent a lot of time “in front of the counter” discussing the merits of vitamin supplements and cautioning consumers that supplementation is not innocuous, despite aisles and aisles of products for self-selection. Many people take the view of vitamins as a reasonable “insurance policy” for their diet, and a net positive thing they can do to support healthy living. And for most of my career, I viewed vitamin supplementation without any clear medical need as producing “expensive urine” for the user, but unlikely to cause much harm. But as more evidence has emerged, my view has changed, and I’m far more skeptical of the net benefits of vitamin supplementation in the absence of a clear deficiency. That position is based on the results of studies that have shown that supplementation is not harmless and has been associated with conditions like heart failure, kidney stones, and overall mortality.
Some of the problems with vitamin supplementation, particularly vitamin D, have emerged when we have identified a correlation between therapeutic levels of a vitamin and a clinical condition. Such is the case for depression where observational research has suggested a potential relationship between vitamin D3 levels and depression. This led to a number of clinical trials that prospectively evaluated vitamin D supplementation using a variety of doses, patient populations, and durations. All but one trial has been negative. Now a new trial provides very persuasive evidence that there’s no there, there.
The trial
The VITAL-DEP trial (Vitamin D and Omega-3 Trial-Depression Endpoint Prevention) was a side trial to the VITAL study, and was published this week. It evaluated the effects of vitamin D3 supplementation on 18,353 adults without depression.
Let’s start with the investigators and the funders. This was a trial primarily funded by the National Institute of Mental Health, with additional grants from other National Institutes. The study supplements were donated by manufacturers. Neither the funders nor any manufacturer had any role in the design or conduct of the study. The lead author was Olivia I. Okereke, from Massachusetts General Hospital and Harvard Medical School. The other investigators are from other US-based healthcare institutions and schools.
The main trial was a double-blind placebo-controlled trial testing vitamin D3 and fish oil supplementation on the prevention of cancer and cardiovascular disease. Participants were randomized into one of four groups, and could receive the active substance, or an identical placebo. The vitamin D3 dose was 2000IU (of cholecalciferol) per day. You were excluded from the trial if you were already taking more than 800IU of vitamin D per day, had cardiovascular disease, and a number of other conditions.
For this side trial, additional exclusion criteria included the presence of clinically relevant depressive symptoms or had features of depression. Other mental health conditions also led to exclusion. After all exclusions there were 18,353 eligible patients.
Patients were followed annually via survey, for a median duration of 5.3 years. Pill adherence (considered to be taking at least 2/3 of the doses) was considered high. A subset of patients had vitamin D levels measured, and the changes were consistent with what was expected.
There were two pre-defined outcomes of interest:
- Risk of depression or clinically relevant depressive symptoms, defined as a new self-report of depression diagnosed by an MD, new treatment for depression, or the presence of clinically relevant symptoms of depression on an annual questionnaire, using a validated survey tool.
- Longitudinal mood scores, measured based on annual questionnaires.
This was a large trial, and the authors calculate it was powered to detect minimally clinically important differences between the groups, if any existed. All participants were followed until the occurrence of the end point, death, or the end of the trial. 90.5% of participants finished the trial, and the two groups were well balanced with no meaningful differences between them. Women made up 49% of the population, 17% were minorities, and 19% were Black.
The results
There were 609 cases of depression or depressive symptoms in the vitamin D3 group and 625 cases in the placebo group. The curves tell the story here: Yes there are two lines, but they are virtually superimposed in (A) below:
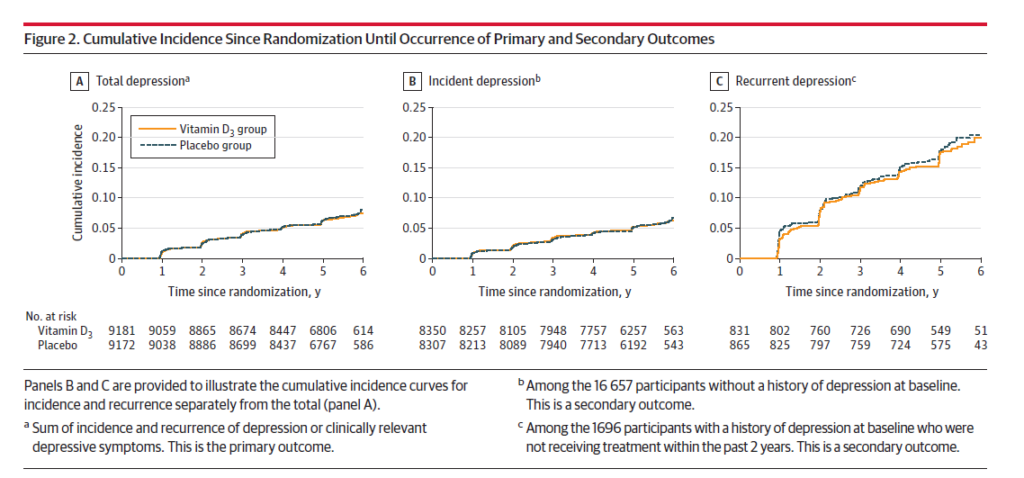
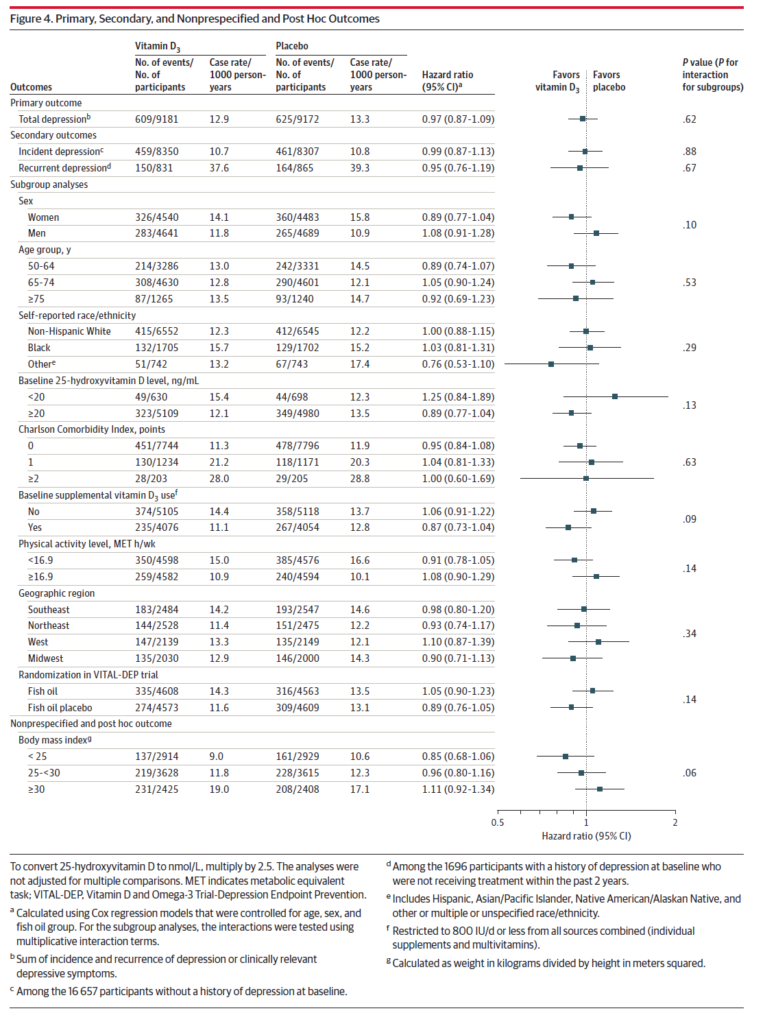
Across every group (age, ethnicity, baseline D3 use, geography, BMI), there was no effect observed with vitamin D supplements:
Should we stop doing trials with vitamin D?
The evidence for supplementation with vitamin D is unconvincing for the prevention of most medical conditions. However, given the low cost, and relative lack of side effects, vitamin D supplementation at modest doses is safe and has not been shown to be harmful. This large randomized controlled trial provides compelling evidence that vitamin D supplementation has no effect on the risk of depression (or depressive symptoms) or its recurrence in adults aged 50 or older. There is no longer an argument to be made that “more research is needed”. This study is probably enough.
The repeated failure of vitamin D in prospective trials raises the question as to whether or not these large trials can be justified financially with public dollars. On one hand, the compelling results could help patients and physicians make better treatment decisions about vitamin D, if those decisions are influenced by evidence. There is also continued public interest in vitamin D consumption, driven in large part by the supplement industry. Given the limited research dollars available and the number of public health issues that would benefit from research, it would seem that industry should be funding this research. But in the absence of requiring supplement manufacturers to provide persuasive evidence to justify marketing their products, it falls on governments and other researchers to do these types of studies. For that reason, we should be grateful for this study which provides conclusive evidence against the use of vitamin D for the prevention of depression.

